PPI Unit I 2015
| Site: | University of Edinburgh Moodle |
| Course: | Patient and Public Involvement [2015-2016] [SEM 2] |
| Book: | PPI Unit I 2015 |
| Printed by: | Guest user |
| Date: | Tuesday, 27 January 2026, 9:53 AM |
Description
PPI 2015 Unit I
1. Introduction
Unit I The origins and evolution of patient and public involvement in research: theory, policy and principles
This unit will introduce the student to the principles and practice of involving patients and the public in research. We will start with definitions of patient and public involvement in research and examine the principles which guide its practice. We will explore the social, theoretical and policy drivers which have led to its adoption and consider what benefits are claimed to arise from involving patients and members of the public in research.
Aims
Unit I has been designed to offer hands-on opportunities to reach an understanding of the rationale for and nature and scope of patient and public involvement in research. This includes the skills to
- Define patient and public involvement in research and public engagement
- Identify the proposed benefits of patient and public involvement in research
- Critically appraise the theory and values underpinning patient and public involvement in clinical research
- Reflect on personal assumptions/preconceptions about patient and public involvement in research
Learning outcomes
After completion of this unit, course participants should have reached the following learning outcomes:
-
Critically appraise the theory and key principles of patient and public involvement in clinical research
-
Critically assess their own practice/place of work in relation to the level of patient and public involvement in research
1.1. Profile: Course leader
 |
Dr Allison Worth is Patient and Public Involvement Advisor for the Edinburgh Clinical Research Facility at the University of Edinburgh. Her role is to support research staff and members of the public to make patient and public involvement effective and enjoyable for all. Allison has a background in nursing and is an experienced healthcare researcher and educator. Her research focus has been qualitative research trying to understand and improve the experiences of patients with long term and life-limiting conditions. Allison looks forward to meeting you on Wednesday, January 13th at 6 pm (GMT) in an Adobe Connect live online welcome session. |
2. Overview
Features
Unit I runs over two weeks (Jan 11th - Jan 24th) and is organised into the following 2 lessons:
- Lesson 1 Patient and Public Involvement in Research: key concepts and definitions
- Lesson 2 Underpinning principles and values
Each lesson is comprised of a selection of partner readings, written content and independent activities. There is a Unit I discussion board which includes 2 Thought questions for collaborative discourse.
As you may know from the course planner, there are a number of key dates and action points for you to be aware of in weeks 1 & 2:
Key dates
Adobe Connect
- Wednesday, Jan 13th (6 - 7 pm): Online lecture with Dr. Allison Worth
- Topic: Introduction to Patient and Public Involvement in Research and review of assignments
- Wednesday, Jan 20th (6 - 7 pm): Online lecture with Dr. Allison Worth
- Topic: The origins and principles of patient and public involvement in research
Adobe Connect URL: http://edinburghcrf.adobeconnect.com/ppi/
Your tasks
Please refer to the Unit I checklist
[Checklist pops up in a separate window]
3. Core readings & resources
For core readings and resources, please refer to the Resource list on the Moodle course homepage (from the menu list on the left-hand side of the page).
4. Lesson 1 Key concepts & definitions
Partner readings
For Unit I partner readings, please refer to the Resource list on the Moodle course homepage (from the menu list on the left-hand side of the page).
Topics
- Definitions:
- patient and public involvement in research
- public engagement
- Proposed benefits of involvement
Introduction
In this lesson, we will look at the meaning of "patient and public involvement" and "public engagement" with plenty of real-life examples. It will help you if you have completed the section 4.1 Your turn activity before the Adobe Connect lecture (Wednesday Jan. 13th, 6-7 pm).
4.1. Your turn: Read
Task I. Read
Please read the guides to patient and public involvement for researchers and the public on the Wellcome Trust Clinical Research Facility web pages before the Adobe Connect lecture on Wednesday Jan. 13th (6-7 pm).
4.2. Your turn: Expert guest lecture
Task I. Join online expert guest lecture
 |
Presenter: Dr Allison Worth Topic: Introduction to patient and public involvement in research, and review of assignments Date: Wednesday, January 13th 2016 Time: 6 - 7 pm (GMT) Location: http://edinburghcrf.adobeconnect.com/ppi/ This session will be recorded. |
4.3. Lesson 1a Definitions
What is patient and public involvement in research?
INVOLVE defines it as “research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them”. It might include: involving people in determining research priorities for a particular condition; members of the public being co-applicants on a research grant; advising on study materials; contributing to data collection, analysis and reports.
What is the difference between a ‘patient’ and a ‘member of the public’?
Usually, if you want to involve someone with a particular health condition, they will be referred to as a patient, but members of the public can provide a generic lay perspective on research. Family members of people with a particular illness or parents may also have valuable perspectives.
What is public engagement?
Public engagement is about communicating what we do to the public. This might include our research findings, explaining the value of a study for health and getting people interested in science. It is related to, and overlaps with, patient and public involvement – people who understand the benefits of research are more likely to want to be involved. We will return to the topic of public engagement in week 7 in more detail.
Illustrative examples of these topics will be discussed.
4.4. Stop & think
Stop & think
After the week 1 lecture and with the help of the Lesson 1 partner readings complete the questions below.
|
Stop & think Q1. What is the difference in perspective between a ‘patient’ and a ‘member of the public’ and what they might bring to research? Q2. Can you explain the relationship between patient involvement in research and public engagement? |
4.5. Lesson 1b Assumptions & misconceptions
There are some tensions in the patient and public involvement field.
Assumptions:
- Patients and the public bring a different, complementary kind of expertise to a research team.
- Patient and public involvement results in research that is more relevant to the needs of patients.
- The patient/lay viewpoint will be welcomed by researchers.
- Patients want to become involved in this way.
- PPI is a moral imperative.
- Patients/the public assume that research leads to better health care (of course, we hope it does, but it may not be as rapid as they expect).
Misconceptions:
- Recruiting patients as participants or interviewing patients as part of a study is involving them.
- Healthcare researchers have all the necessary knowledge and expertise for a clinical trial without involving patients.
- Patients can be placed on a Trial Steering Committee without any preparation/training and treated the same as any other committee member
4.6. Lesson 1c Benefits
It is claimed that patient and public involvement improves the quality, relevance and impact of research.
|
“No matter how complicated the research, or how brilliant the researcher, patients and the public always offer unique, invaluable insights. Their advice when designing, implementing and evaluating research invariably makes studies more effective, more credible and often more cost efficient as well”. Professor Dame Sally Davies, Chief Medical Officer |
These are some of the proposed benefits:
- It ensures research is relevant to the needs of patients and society
- It improves study design
- It improves recruitment and retention
- It improves communication between researchers and participants and between researchers and the public
4.7. Your turn: Thought question 1
 Task I. Join the Unit I Thought discussion
Task I. Join the Unit I Thought discussion
Please join us in the Unit I Thought discussion for Thought question 1.
Thought question 1
Let’s talk about lay and professional expertise. Healthcare researchers sometimes claim, because of their clinical activities, they can represent patient views and understand their needs, so they don’t need to involve them in their research. We believe this is a misconception. Whose side are you on and why? We'd like to know!
We look forward to reading and discussing your views.
4.8. Your turn: Reflect
Task I. Reflect
After the lecture, use your reflective diary to express your reaction to what you have learned. What is your initial reaction to the concept of patient and public involvement in research? Critically assess the situation in your own workplace – how far does your workplace involve patients/the public and what more could be done?
5. Lesson 2 Principles & values
Partner readings
For partner readings, please refer to the Resource list on the Moodle course homepage (from the menu list on the left-hand side of the page).
Topics
- Origins of patient and public involvement in research
- Underpinning principles of patient and public involvement in research
- Levels of involvement
Introduction
In this lesson, we will examine where patient and public involvement came from and why it has gained such momentum. We'll focus on the values and principles which drive patient and public involvement in research. Finally, we'll look at varying degrees of depth of patient and public involvement in research.
5.1. Your turn: Expert guest lecture
Task I. Join online expert guest lecture
 |
Presenter: Dr Allison Worth Topic: The origins and principles of patient and public involvement in research Date: Wednesday, January 20th 2016 Time: 6 - 7 pm (GMT) Location: http://edinburghcrf.adobeconnect.com/ppi/ This session will be recorded. |
5.2. Lesson 2a Origins
The origins of patient and public involvement can be found in the 1970s, when consumerism in society gained strength as a movement, challenging traditional power relationships. It was characterised by enthusiasm for more empowering and participatory forms of social action and decision-making, and for greater openness and accountability on the part of public sector organisations.
By the 1990s, this had begun to influence healthcare policy and research. It was claimed that the interests of patients as health service users and as taxpayers would be better served if they could influence the design and conduct of research. By 2001, the UK Government was recommending that ‘participants or their representatives should be involved wherever possible in the design, conduct and reporting of research’.
Another possible influence is the growth in public scepticism of research following the Bristol and Alder Hey scandals. Patient and public involvement in research now has influential champions such as Dame Sally Davies and Prof Ian Chalmers.
Underpinning values
Ives et al. identify two categories of motivation for patient and public involvement in research:
-
Pragmatic/outcome oriented: PPI is a means to an end i.e. it improves the relevance and quality of research.
-
Ideological/process oriented: PPI is an end in itself i.e. it reflects democratic and ethical principles.
There’s a third motivation that is not value-driven: researchers involve patients and the public because they are required to, usually by the grant-funding body they are applying to.
Let’s examine these in more detail.
-
The pragmatic argument: patients’ experiential knowledge of illness and health care will be beneficial to research, therefore it is useful.
-
The moral imperative: patients/the public have a right to be involved in publicly funded research that may impact on their health and services; it improves the transparency and accountability of research.
-
Ticking the patient and public involvement box to get a grant, without necessarily believing in it.
|
|
Stop & think Q1. How might these three different motivations for involving people (it helps my research; it’s a right and a duty; I’ll do it if it helps me get my grant) influence how patient and public involvement is conducted? |
5.3. Your turn: Thought question 2
 Task I. Join the Unit I Thought discussion
Task I. Join the Unit I Thought discussion
Please join us in the Unit I Thought discussion for Thought question 2.
Thought question 2
Consider the the claims by Ives et al below and critically appraise one. Explain your view.
- A fully democratic, cooperative model of patient and public involvement is an ideal that can never truly flourish, because the concept is not internally coherent.
- Once a patient or member of the public undergoes training, and becomes familiar enough with research to be substantially involved, their ‘lay’ status is compromised.
We look forward to your thoughts!
5.4. Lesson 2b Levels of involvement
There are different levels of patient and public involvement ranging from informing people about what researchers do, to collaboration and empowerment, as illustrated here:
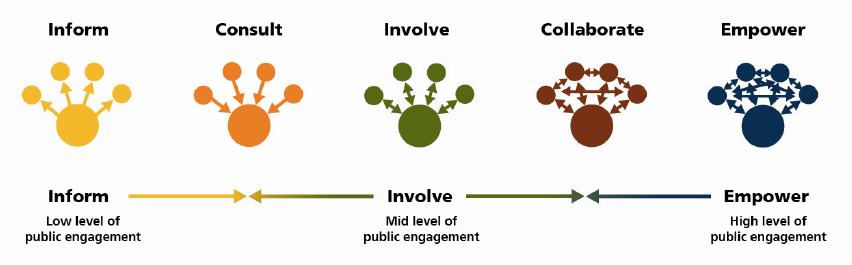
Source: Adapted from the International Association for Public Participation (IAP2 Federation) Public Participation spectrum, 2015
Researchers often claim they are ‘involving’ people by informing the public about the results of a study through a lay summary or a media output. It’s a one-way communication based on professional expertise, whereas true involvement requires researchers to listen and act on the patient/public view in the design and conduct of their research. The ‘empowerment’ end of the continuum means the research is ‘user-led’. Patients and the public are in charge of the research, including managing a budget, which represents a radical culture shift. An effective patient and public engagement strategy for a research study or programme might require activity at all levels of the continuum.
5.5. Your turn: Reflect
 Task I. Reflect
Task I. Reflect
In your reflective diary consider:
- Now that you know more, what is your personal and professional view on whether patient and public involvement in a clinical trials setting is a) a good idea in principle, and/or b) practical and achievable in practice?
- What are your own values regarding why you would (or wouldn’t) involve patients/the public in research?
- What level of involvement have you achieved in your own workplace and what can you aspire to?
Be honest now…
Later we will discuss the subject in an online tutorial with Allison Worth on Thursday Jan. 28th (6-7pm).
6. Unit I summary
Think back on the Unit I lessons and activities - what have we learned?
|
Summary & Conclusions Unit I You now have an understanding of the nature of patient and public involvement in research from policy, theoretical and clinical/managerial perspectives. You have been encouraged to think reflectively on your own stance regarding the purpose and value of patient and public involvement in research. This means you are ready to tackle Unit II where we will consider in greater detail the practicalities and barriers and facilitators to making patient and public involvement work in practice. |
Consider the Unit I learning outcomes-have you achieved them?
You have now completed Unit I. Unit II will be released in week 3.